The Following Ground State Electron Configuration Violates___________.
Which of the. The ground state electron configuration of a Mn2 ion is 1s22s22p63s23p63d5.
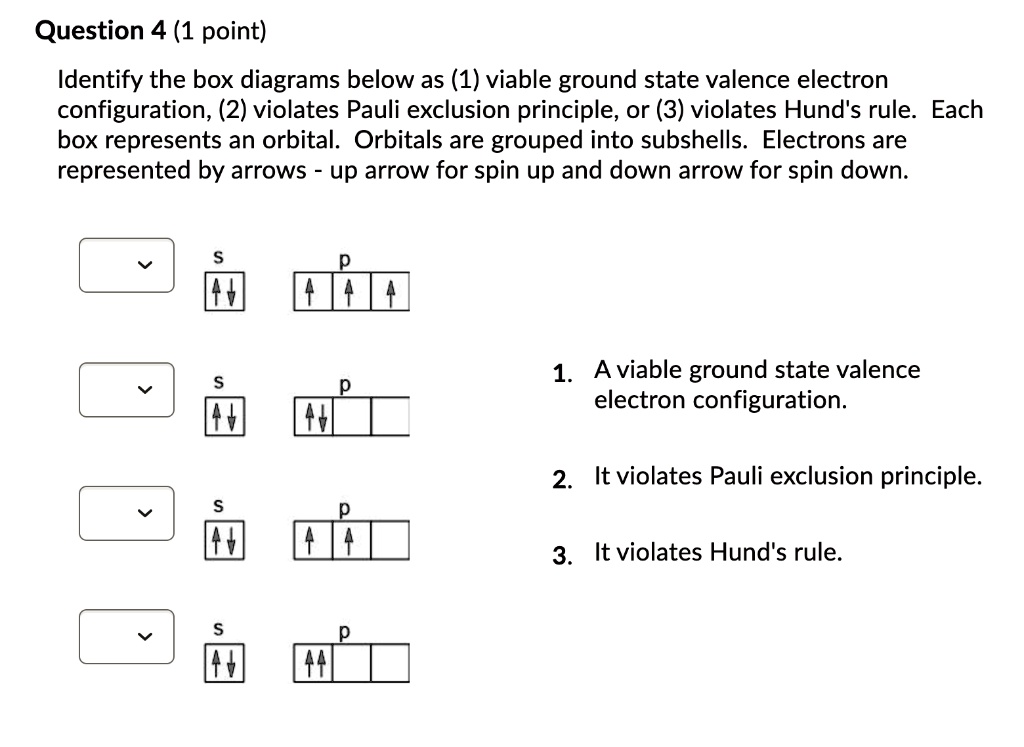
Solved Question 4 1 Point Identify The Box Diagrams Below As 1 Viable Ground State Valence Electron Configuration 2 Violates Pauli Exclusion Principle Or 3 Violates Hund S Rule Each Box Represents An Orbital
This configuration cannot be the ground-state electron configuration for a Mg atom because it violates Hunds rule.

. Which element has the ground state electron configuration Kr5s²4d¹⁰5p³. The following do not represent valid ground-state electron configurations for an atom either because they violate the Pauli exclusion principle or because orbitals are not filled in order of increasing energy. The following do not represent valid ground-state electron configurations for an atom either because they violate the Pauli exclusion principle or because orbitals are not filled in order of increasing energy.
1s 2s 2p C. Indicate which of these two principles is violated in each example or whether both or neither are violated. So if we talk about electronic config and rule violations we must go for ground states exceptional cases are ceCu ceCr and ceMo.
An excited state configuration is meant only when the particular atom gets ready or excited for forming a bond. Paramagnetic with 5 unpaired electrons. Indicate which of these two principles is violated in each example.
So the policy exclusion principle states that no two electrons can have the same for quantum numbers. Which of the following ground state electron configuration represent a violation of the Aufbau Principle. 1s 2s 2p b.
This configuration is the ground-state electron configuration for a Mg atom. Paramagnetic with three unpaired electrons e. According to Hunds rule of maximum multiplicity Pairing of electrons in the orbitals belonging to same subshell ie.
Indicate which of these two principles is violated in each example or whether both or neither are violated. Which of the following can behave like a wave and also like a stream of particles. Which electron configuration violates Hunds rule.
The Pauli exclusion principle. Chemistry questions and answers. A Te B Pb C Sb D Bi E Sn.
Classify the following elements as main group elements transition metals or inner transition metals. This configuration cannot be the. Which electron configuration represents a violation of Hunds rule for an atom in the ground state.
If we look at part A in the s orbitals there can only be um theres only 11 orbital and it can ho. 1s 2 2s 2 2p3. Which of the following has the electron configuration 1s²2s²2p⁶3s²3p⁶3d³.
1s 2 2s 2 3s 1. B Mn³ C Cr² D Sc² E V² E. Of the following transitions in the bohr hydrogen atom the _______ transition results in the emission of the lowest-energy photon.
What is the correct electron configuration for a ground-state atom with 7 electrons. The following do not represent valid ground-state electron configurations for an atom either because they violate the Pauli exclusion principle or because orbitals are not filled in order of increasing energy. This configuration cannot be the ground-state electron configuration for a Sr atom because it violates Hunds rule.
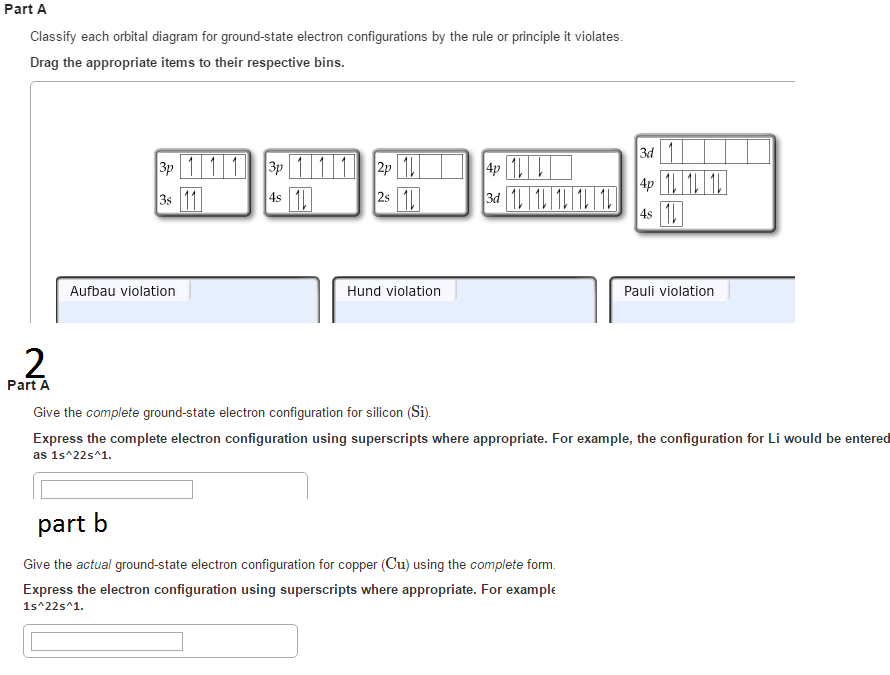
According to the given Diagram in option C Aufbau principle is violated as the electron enters to 3 d orbital before 4 s and it also violates the Hunds rule as. Drag the appropriate items to their respective bins. This configuration cannot be the ground-state electron configuration for a Mg atom because it violates the Heisenberg uncertainty principle.
The following do not represent valid ground-state electron configurations for an atom either because they violate the pauli exclusion principle or because orbitals are not filled in order of increasing energy. Classify each orbital diagram for ground-state electron configurations by the rule or principle it violates. Paramagnetic with one unpaired electron d.
The ground-state electron configuration of a Mn2. The electron configuration below violates a. This configuration is the ground-state electron configuration for a Sr atom.
1s 2s 2p d. Indicate which of these two principles is violated in each example or whether both or neither are violated. Paramagnetic with five unpaired electrons b.
A Ne3s²3p3d_ b Xe6s³ c 1s²3s. Paramagnetic with two unpaired electrons. Give the ground-state electron configuration for silicon Si using noble-gas shorthand.
In other words no more than two electrons can occupy the same orbital and two electrons in. Degenerate orbitals does not take place until each orbital belonging to that subshell has got one electron each ie. Indicate which of these two principles is violated in each example.
The correct option is A. The following ground state electron configuration violates 1 1 1 1 1 1s 2s 2p A Heisenbergs Uncertainty principle B Hunds Rule C the Aufbau principle D the Pauli Exclusion principle E None of these. Indicate which of these two principles is violated in each example or whether both or neither are violated.
All of the following ground-state electron configurations are correct except. Like boron does it with 3 fluorine atoms to form ceBF3. The following do not represent valid ground-state electron configurations for an atom either because they violate the Pauli exclusion principle or because orbitals are not filled in order of increasing energy.
The following do not represent valid ground-state electron configurations for an atom either because they violate the pauli exclusion principle or because orbitals are not filled in order of increasing energy. This configuration cannot be the ground-state electron configuration for a Sr atom because it violates the Pauli exclusion principle. Correct option is B Paulis Exclusion Principle states that no two electrons in the same atom can have identical values for all four of their quantum numbers.
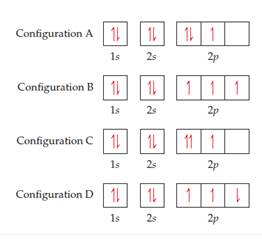
Get Answer Four Possible Electron Configurations For A Nitrogen Atom Are Transtutors
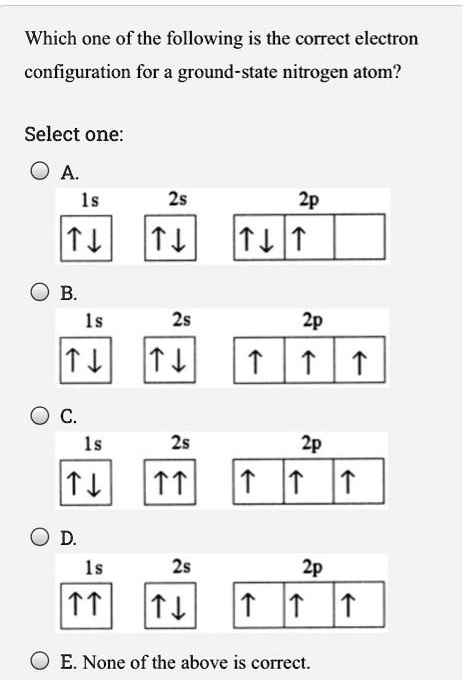
Solved Which One Of The Following Is The Correct Electron Configuration For A Ground State Nitrogen Atom Select One 2s 2p 4 T 2p 25 2p 15 2p E None Of The Above Is
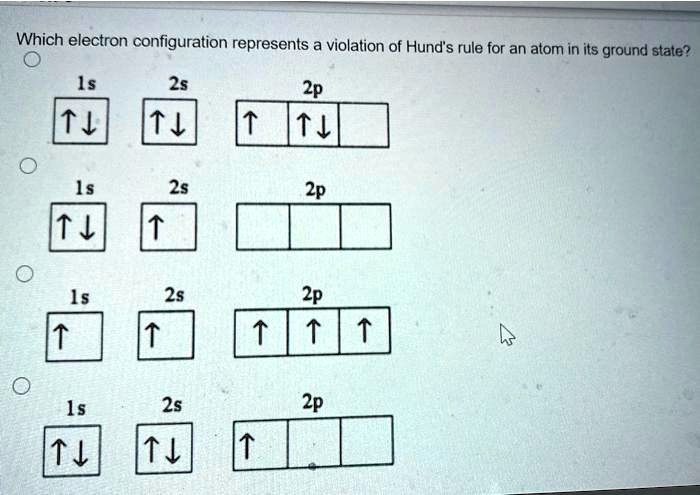
Solved Which Electron Configuration Represents A Violation Of Hund S Rule For An Atom In Its Ground State Ls 25 2p 1l 2s 2p 25 2p 15 2s 2p
Solved Classify Each Orbital Diagram For Ground State Chegg Com
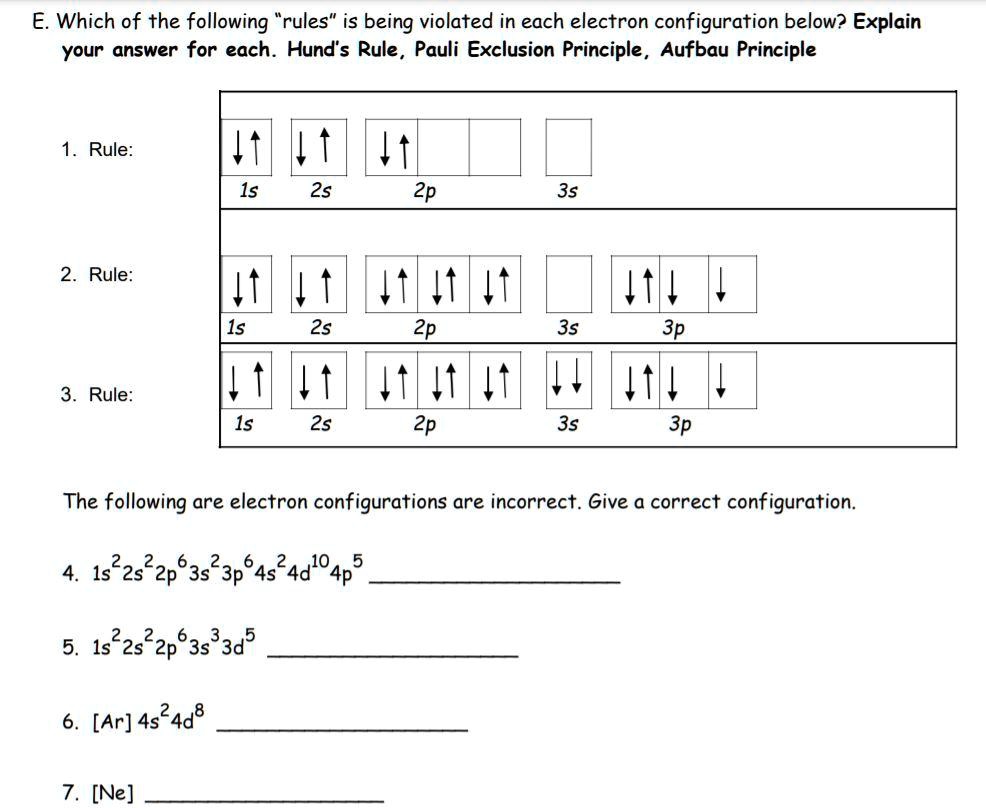
Solved E Which Of The Following Rules Is Being Violated In Each Electron Configuration Below Explain Your Answer For Each Hund S Rule Pauli Exclusion Principle Aufbau Principle Rule 1s 2s 2p 3s
What Rules And Principles Are Violated In The Electronic Configuration Of Chromium Quora
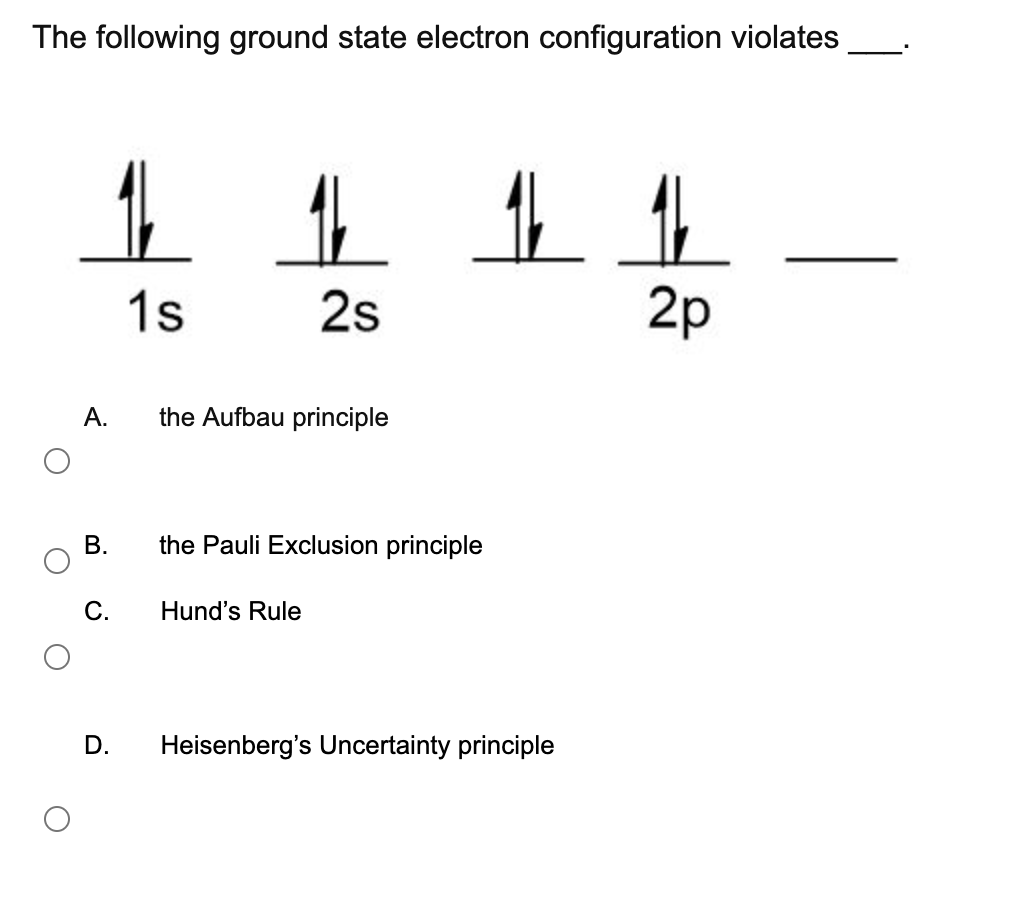
Solved The Following Ground State Electron Configuration Chegg Com
What Is The Electronic Configuration Of Halogen Family Quora
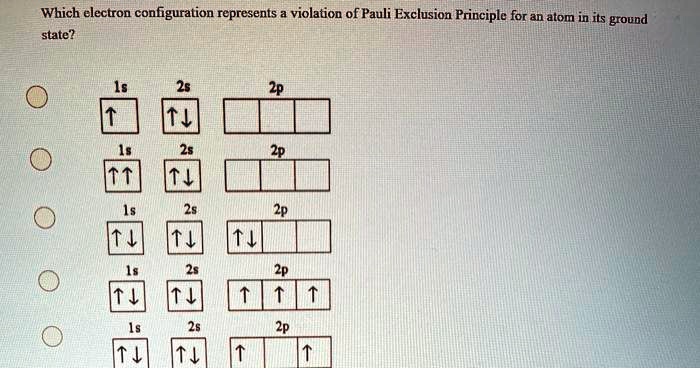
Solved Which Electron Configuration Represents Violation Of Pauli Exclusion Principle For An Atom In Its Ground State

Solved State Hund S Rule Would And Of The Following Ground Chegg Com

Solved Four Possible Electron Configurations For A Nitrogen Chegg Com
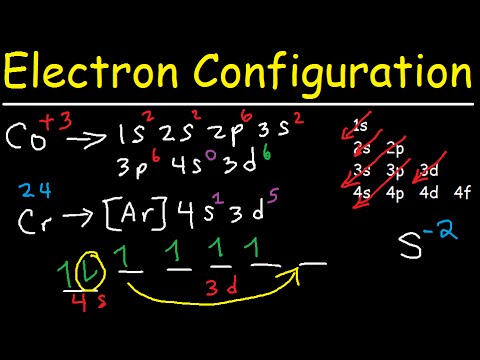
Electron Configuration Quick Review Youtube

Solved The Following Ground State Electron Configuration Chegg Com

Solved Be Sure To Answer All Parts Portions Of Orbital Chegg Com
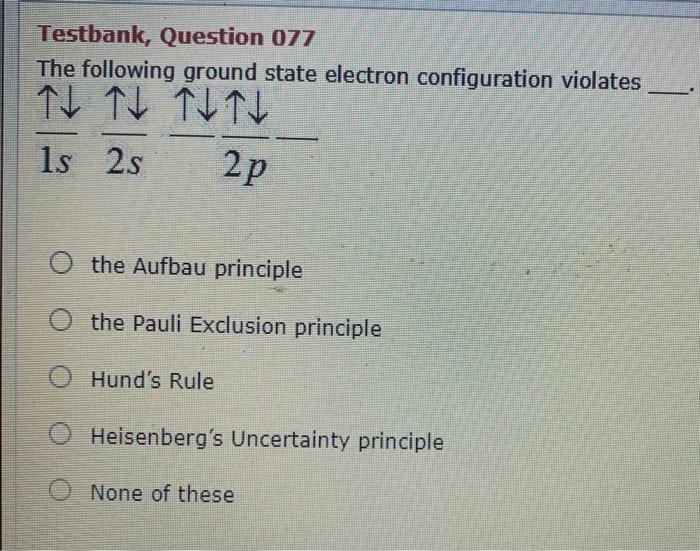
Solved Testbank Question 077 The Following Ground State Chegg Com

Solved 8 The Following Ground State Electron Configuration Chegg Com
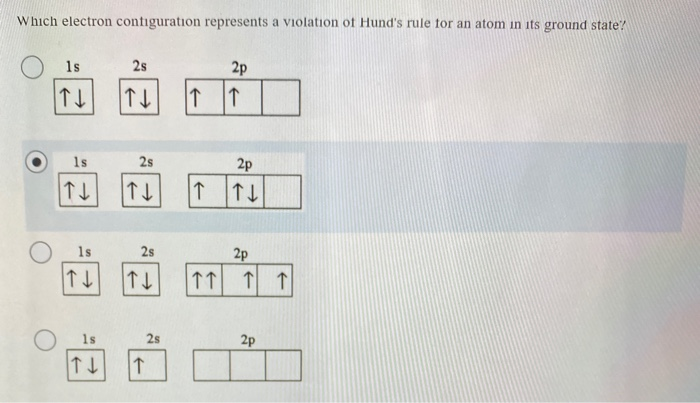
Solved Which Electron Configuration Represents A Violation Chegg Com
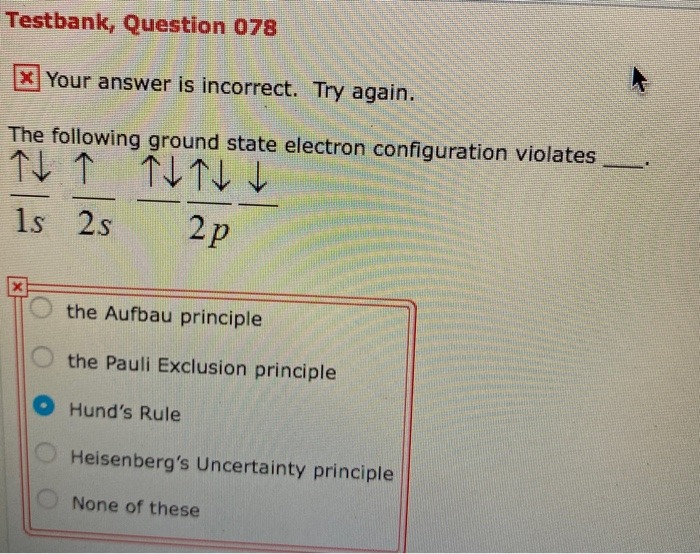
Solved Testbank Question 078 X Your Answer Is Incorrect Chegg Com

Portions Of Orbital Diagrams Representing Ground State Electron Configurations Of Certain Elements Are Shown Below Use These Homeworklib
Comments
Post a Comment